By Alec Go
The Department of Health (DOH) announced that individuals who were administered Sinopharm COVID-19 vaccines in their primary dose series will be eligible to receive booster shots.
According to the DOH, the vaccines of Sinopharm, AstraZeneca, Pfizer-BioNTech, and Moderna can be used as booster dose for Sinopharm recipients.
DOH USec. Vergeire: Maari nang iturok ang Sputnik Light bilang booster shot matapos ang primary series doses. Paalala lamang po na ang eligible pa lamng sa ating booster shots ay mga 18 years-old pataas.
— PTVph (@PTVph) January 28, 2022
In the Jan. 28 Palace briefing, Health Undersecretary Maria Rosario Vergeire said an emergency use authorization has been issued by the Food and Drug Administration, together with the guidance from the National Vaccination Operations Center.
The DOH also approved the use of Gamaleya’s Sputnik Light vaccine in the country’s COVID-19 booster administration.
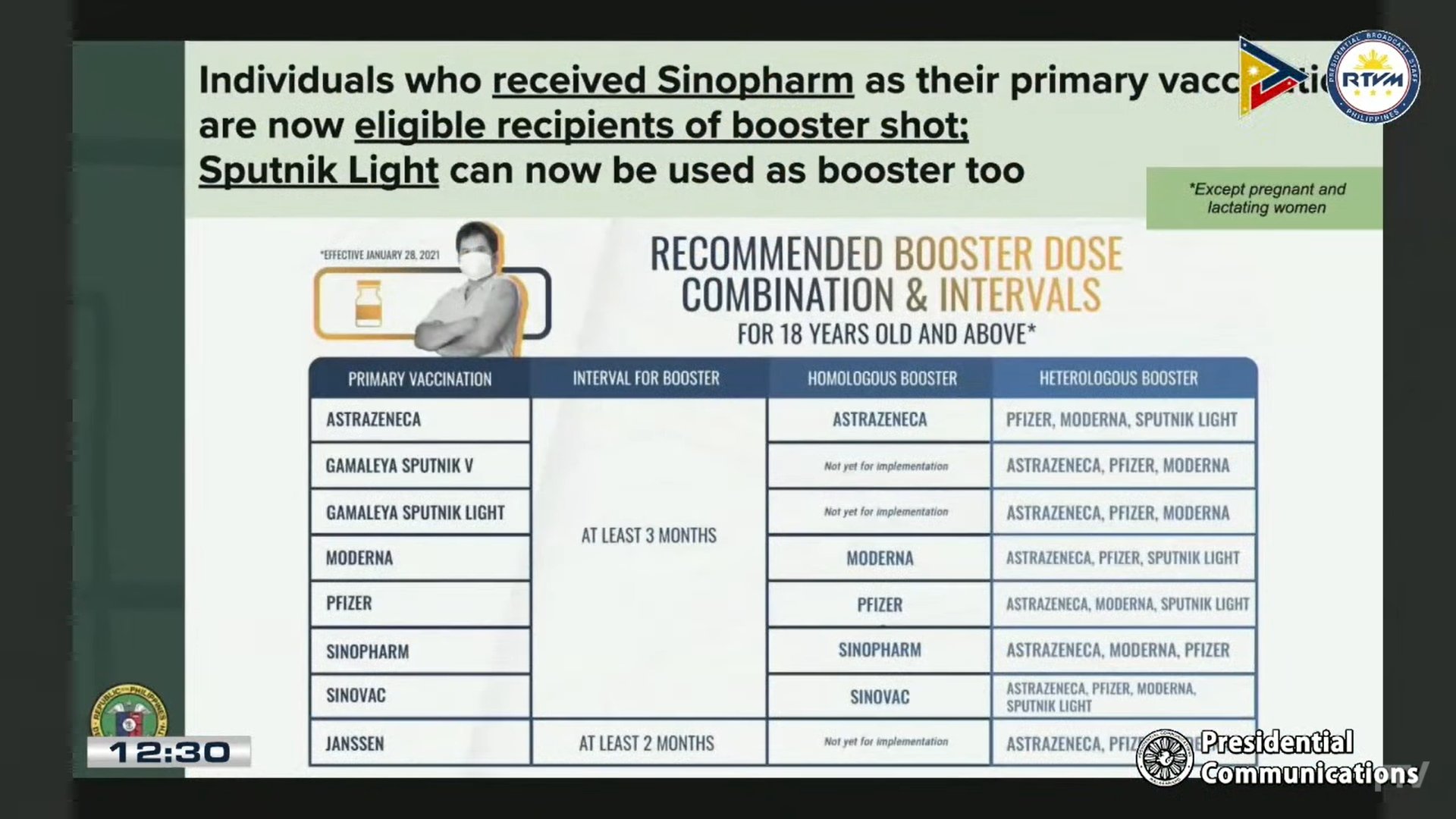
“Ang eligible pa lamang sa ating booster shots ay ang mga 18 years-old at pataas. Amin din po nililinaw na ang mga nabanggit na booster doses ay nakadepende pa rin sa available vaccines,” she said.
“Nais pong bigyang diin ng DOH na hindi puwedeng i-booster sa mga buntis at mga nagpapasuong ina ang Sinopharm at Gamaleya Sputnik,” she added.
Based on the agency’s infographic, Sputnik Light may be used as a booster for those who have received AstraZeneca, Moderna, Pfizer, and Sinovac jabs in their primary series.
