The Department of Health (DOH) and the Food and Drug Administration (FDA) on Thursday (April 23) assured the efficacy and safety of Covid-19 vaccines in preventing death and hospitalization amid some incidents of adverse events after vaccination.
The DOH and FDA reported that almost all cases of adverse events following immunization (AEFIs) were vaccinees who experienced mild side effects, and only 1.5% of the AEFIs were in serious condition as of April 10.
The health agencies also clarified that all reported serious AEFIs were later discovered to be “coincidental and totally unrelated to the vaccine,” after an assessment conducted by the National AEFI Council (NAEFIC), an independent group of experts formed to probe AEFI reports.
“From the start of our vaccination period to April 10, only 1.5% of the total AEFIs were serious cases. This means that for every 1,000,000 vaccinated, less than 0.04% may experience serious AEFI cases,” FDA Director-General Enrique Domingo said.
“But it does not necessarily mean that the vaccine causes the events. This risk is much lower than the fatality of the Covid-19 infection in our country which has a 1.69% fatality rate,” Domingo added.
They also clarified that among the normal signs that the body is developing protection against Covid-19 after vaccination are headache, fever, pain in the injection site, malaise, fatigue, muscle pain, and increased blood pressure.
It added that all adverse events are “properly attended to” and comprehensive pre- and post-vaccination screening are in place.
“All vaccination sites have holding stations where vaccinated individuals are closely monitored for any adverse reaction to the vaccine. The government also set up an indemnification fund to ensure the welfare of patients who will experience serious adverse events,” DOH Secretary Francisco Duque III said.
Duque, who is included in priority group A2 or senior citizens, received his first jab of CoronaVac on April 23. He underscored the efficacy and safety of the vaccines and said that the vaccine rollout will save more lives.
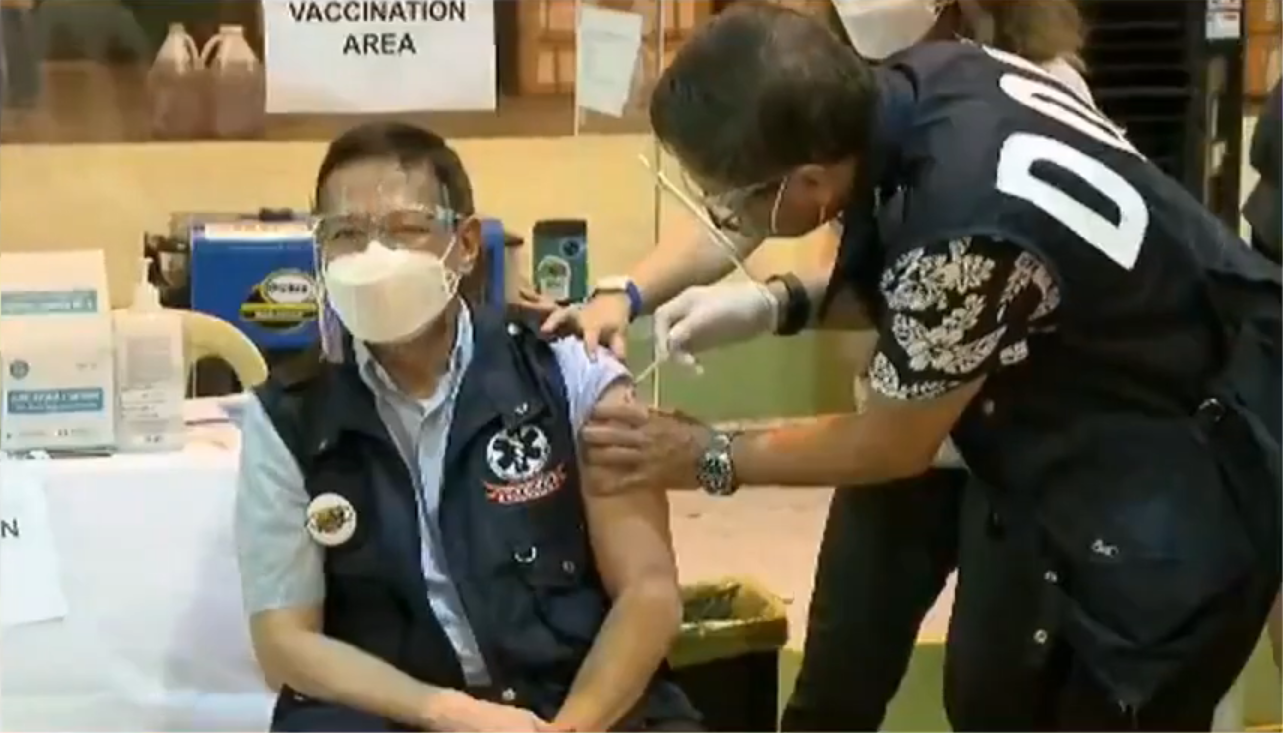
“As I receive my dose of the Covid-19 vaccine today, I invite everyone to do the same, and choose to be protected. Let us all take part in protecting public health, and let us be in unison in spreading one message: that vaccines are safe, and vaccines are effective,” Duque said.
Meanwhile, the government assured that the vaccine cluster will do its part to secure sufficient vaccine doses to cover the inoculation of 70 million Filipinos. – (DOH, FDA) /AG-jlo
